Purify transfection-grade plasmid DNA from bacteria using a fully automated, magnetic bead-based custom protocol with the Wizard MagneSil Tfx™ System on the CyBio FeliX liquid handler.
Kit: Wizard MagneSil Tfx™ System, 4 x 96 preps (Cat.# A2380)
Analyses: UV absorbance & agarose gel electrophoresis
Sample Type(s): Bacteria from overnight cultures
Input: 0.5ml to 1ml of bacterial cultureMaterials
Required:
- Wizard MagneSil Tfx™ System (Cat.# A2380)
- MagnaBot® FLEX 96 Magnetic Separation Device (Cat.# VA1290)
- 2.0ml Deep Well Plates (Sterile) (Cat.# AS9307)
- 100% Isopropanol (molecular biology grade)
- 80% Ethanol (molecular biology grade)
- Nunc® 2.0ml Deep-Well Plate Adapter (QInstruments, Cat.# 2016-1151)
- Reservoir, 4 column, polypropylene, 73ml (Agilent, Cat.# 201308-100)
Analytik Jena Equipment and Consumables:
- CyBio FeliX Basic Unit with Enclosure (Cat.# OL5015-24-100)
- CyBio FeliX Extraction Set (Cat.# OL5015-25-120)
- CyBio TipRack 96/1000µl; PCR-certified, pre-sterilized, filter (Cat.# OL3811-25-939-F)
- Protective Plate, 532 Pieces (Cat.# OL3317-25-126)
Method:
Sample Preparation
Aliquot 0.5ml to 1ml of cultured bacteria into 96-well plates (round bottom 2ml deep well) and centrifuge at 4,500 x g for 30 minutes to pellet bacterial cells. Manually remove and discard the supernatant.
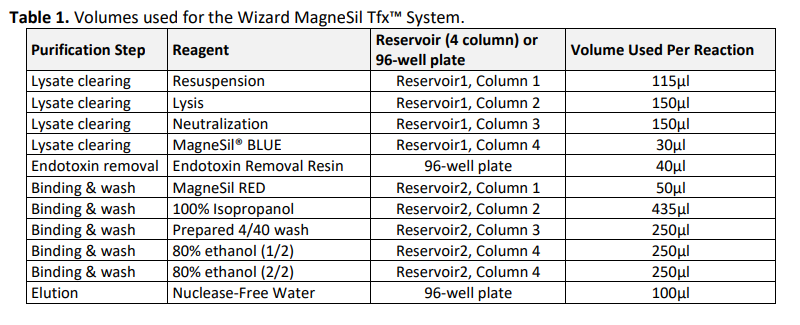
Wizard MagneSil Tfx™ System and Instrument Setup
- Endotoxin removal plate - Add 40µl of Endotoxin Removal Resin to a new 96-well plate for each sample to be processed. Use the same well positions as the sample preparation plate.
- Elution plate - Add 100µl of Nuclease-Free Water to a new 96-well plate for each sample to be processed. Use the same well positions as the sample preparation plate.
- Binding mastermix preparation – Mix 50µl of MagneSil® RED and 435µl of 100% isopropanol (plus a 10% overage) per sample into position 1 of a single 4-column reservoir.
- Other kit reagents will be loaded to 4-column reservoirs according to the CyBio FeliX method instructions. See Table 1 for reagent volumes used per reaction.
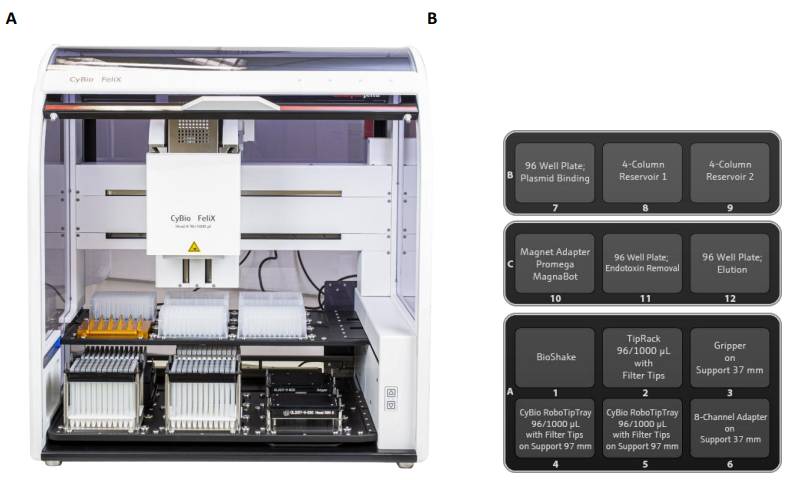
 Instrument Method
Instrument Method
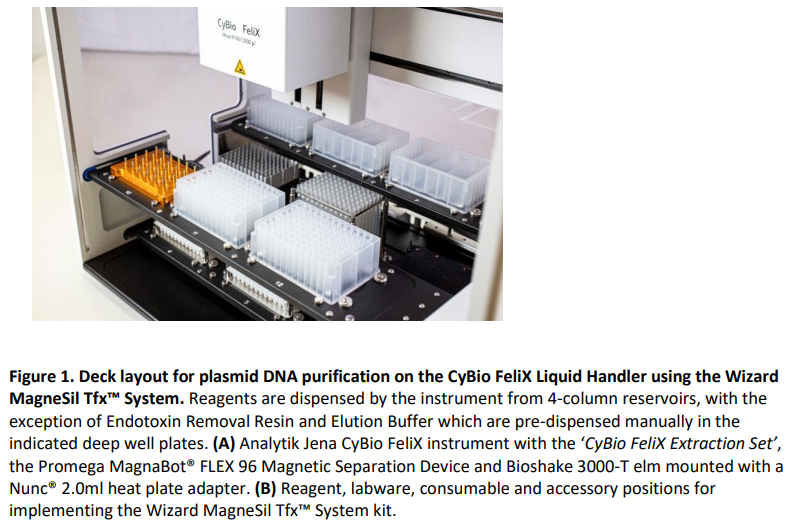
Load and run the CyBio FeliX method “Wizard MagneSil Plasmid R96-1000 (Endotoxin Removal).bms” and follow the on-screen instructions. Instrument and deck layout pictures are shown in Figure 1.
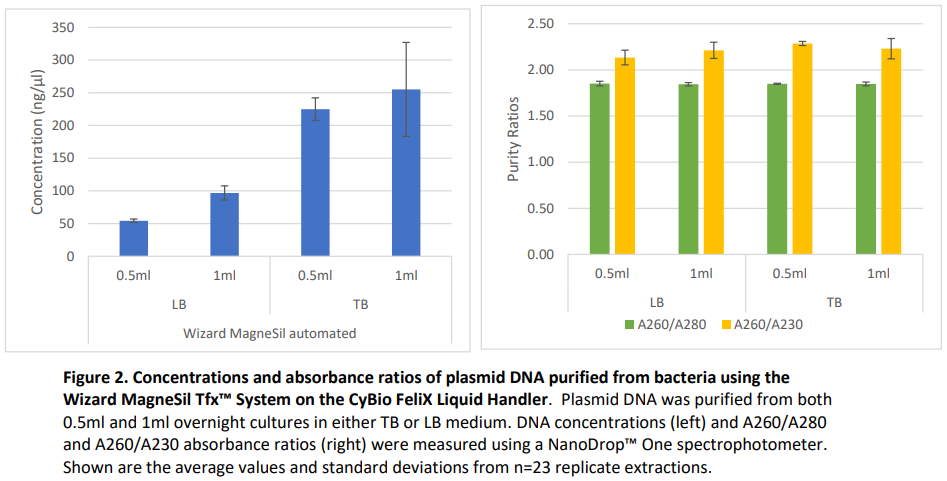
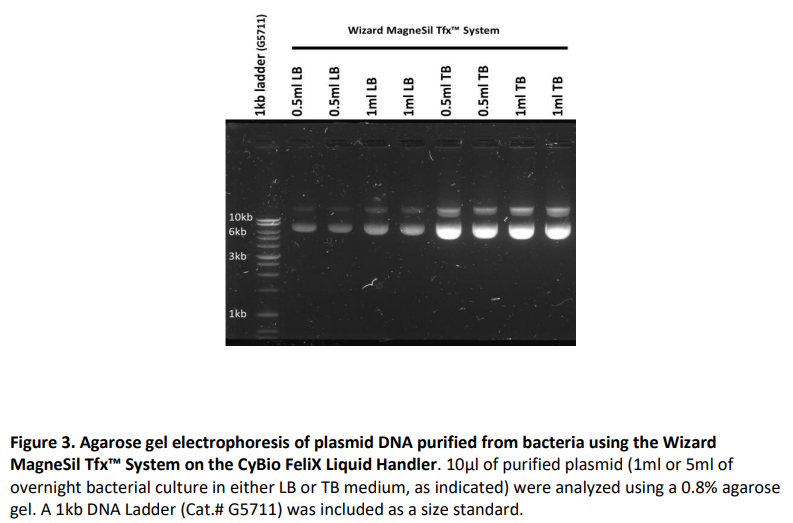
 Results
ResultsPlasmid DNA purifications were performed using the method described above from 0.5ml and 1ml pelleted bacterial cultures. JM109 E. coli bacteria (Cat.# L2005) was transformed with pGL4.50 (Cat.# E131A) and cultured overnight in Terrific Broth medium (TB) or Luria Broth medium (LB). Replicate purifications were performed (N=23). Concentrations and absorbance ratios (A260/A280 and A260/A230) were determined using spectrophotometry (NanoDrop™ One). Plasmid size was visualized using gel electrophoresis. Plasmid DNA was successfully purified from both 0.5ml and 1ml of LB and TB cultured bacteria, respectively. As expected, extractions from TB culture resulted in higher plasmid yields due to the increased density of the overnight culture. Absorbance purity ratios and gel electrophoresis demonstrated that the purified plasmid DNA was of high quality and purity.